Claudio Díaz-García, Elena Moreno, Alba Talavera-Rodríguez...Santiago Moreno & Sergio Serrano-Villar. Fecal microbiota transplantation alters the proteomic landscape of inflammation in HIV: identifying bacterial drivers
Microbiome. 2024
"Fecal Microbiota Transplantation Reduces Systemic Inflammation in HIV: A Step Towards Microbiome-Based Therapies"- Dr. Sergio Serrano Villar -
Summary:
Background: Despite effective antiretroviral therapy, people with HIV (PWH) experience persistent systemic inflammation and increased morbidity and mortality. Modulating the gut microbiome through fecal microbiota transplantation (FMT) represents a novel therapeutic strategy. We aimed to evaluate proteomic changes in inflammatory pathways following repeated, low-dose FMT versus placebo.
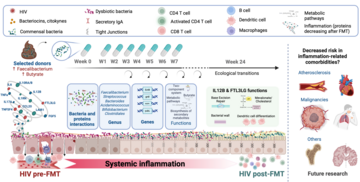
Methods: This double-masked, placebo-controlled pilot study assessed the proteomic impacts of weekly FMT versus placebo treatment over 8 weeks on systemic inflammation in 29 PWH receiving stable antiretroviral therapy (ART). Three stool donors with high Faecalibacterium and butyrate profiles were selected, and their individual stools were used for FMT capsule preparation. Proteomic changes in 345 inflammatory proteins in plasma were quantified using the proximity extension assay, with samples collected at baseline and at weeks 1, 8, and 24. Concurrently, we characterized shifts in the gut microbiota composition and annotated functions through shotgun metagenomics. We fitted generalized additive models to evaluate the dynamics of protein expression. We selected the most relevant proteins to explore their correlations with microbiome composition and functionality over time using linear mixed models.
Results: FMT significantly reduced the plasma levels of 45 inflammatory proteins, including established mortality predictors such as IL6 and TNF-α. We found notable reductions persisting up to 16 weeks after the final FMT procedure, including in the expression of proteins such as CCL20 and CD22. We identified changes in 46 proteins, including decreases in FT3LG, IL6, IL10RB, IL12B, and IL17A, which correlated with multiple bacterial species. We found that specific bacterial species within the Ruminococcaceae, Succinivibrionaceae, Prevotellaceae families, and the Clostridium genus, in addition to their associated genes and functions, were significantly correlated with changes in inflammatory markers.
Conclusions: Targeting the gut microbiome through FMT effectively decreased inflammatory proteins in PWH, with sustained effects. These findings suggest the potential of the microbiome as a therapeutic target to mitigate inflammation-related complications in this population, encouraging further research and development of microbiome-based interventions.
Why do you highlight this publication?
Through the integration of longitudinal data from an independent, controlled clinical trial, we identified the bacteria, genes, and functions that influence inflammation following fecal microbiota transplantation (FMT) from donors selected for their anti-inflammatory microbial signature. Inflammation was measured comprehensively using a panel of over 350 inflammatory proteins. This study not only deepens our understanding of the microbiome's role in people with HIV but also offers potential for designing microbiome-based therapies for other diseases characterized by a pro-inflammatory context.
Publication commented by:
Claudio Díaz-García, Dr. Elena Moreno and Dr. Sergio Serrano-Villar
Infectious Diseases Service
INFECTIOUS DISEASES
IRYCIS